Usage detail
Adhesive materials, modifiers, extruded coatings, etc.
- Overview
- Grade Chart
- Molding methods
- Usage detail
- Product Safety Information
Introduction
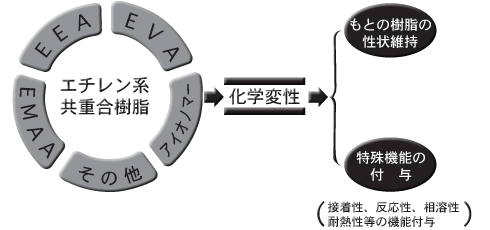
HPR is a collective term for "modified resins," which are the various ethylene-based copolymer resins produced and sold by Dow-Mitsui Polychemicals that have been chemically modified based on technological capabilities cultivated over many years.
When an ethylene-based copolymer resin is chemically modified, the properties of the resin prior to modification are retained, while the resin gains special functions due to the modification.
Features
Handling precautions
- VThe VR and AR series are resins with high moisture absorbency. If residual resin is left in a hopper or bag for an extended period after molding is complete, especially under high humidity, it could cause foaming when next processed due to the absorption of moisture.
Please store any residual resin sealed in a moisture-proof bag. - When processing resins of each series, please observe the maximum processing temperatures listed in the selection guidelines.
- Please avoid keeping resin in an extruder or die for extended periods, as it could cause problems such as altering the resin.
- When shutting down a molding machine after molding, thoroughly purge and replace the inside of the molding machine with polyethylene.
HPR
| Grade | Test item | Melt flow rate | Density | Tensile stress at break |
Tensile breaking strain |
Bending rigidity | Durometer A hardness |
Vicat softening temperature |
Melting point |
|---|---|---|---|---|---|---|---|---|---|
| Measurement method | JIS K7210:1999(190ºC / 2.16 kg load) | JIS K7112 | JIS K7161-1:2014 K7161-2:2014*1 |
JIS K7161-1:2014 K7161-2:2014*1 |
JIS K7106 :1995 |
JIS K 7215 :1986 |
JIS K7206:1999 |
(DSC) | |
| Unit | g/10min | kg/㎥ | Mpa | % | Mpa | - | ℃ | ℃ | |
| VR105-2 | 18 | 980 | 2.3 | 1670 | -*2 | 39 | 40> | -*3 | |
| AR2011 | 8.0 | 950 | 4.2 | 920 | 6 | 66 | 40> | 76 | |
| VS171 | 5.5 | 940 | 17 | 720 | 49 | 93 | 62 | 92 | |
← Swipe →
*1 Tensile test - test piece type and testing speed: JIS K 7161-2 / 1BA / 20
*2 Out of measurable range of the measuring instrument
*3 Without melting point or non-crystalline
·Note: The data above are typical values and cannot be used as standard values.
Usage detail
The following processing methods are applicable to each HPR series.
Table HPR Processing Method Selection Guideline
| Series | Upper limit of processing temperature (°C) |
Extrusion coating |
T-die casting |
Extrusion molding |
Melting kettle melting |
Solvent dissolution (toluene) |
|---|---|---|---|---|---|---|
| VR | 230 | VR105-2 (23℃/15wt%) (40℃/35wt%) |
||||
| AR | 280 | AR2011 | AR2011 |
AR2011 | AR2011 | VR105-2 (23℃/ 5wt%) (40℃/20wt%) |
| VS | 245 | VS171 | VS171 | VS171 |
← Swipe →
The special features and main applications granted to each HPR series are as follows
Table Main Features and Applications
| Series | Base resin | Special Features | Main applications |
|---|---|---|---|
| VR | EVA |
|
|
| AR | EEA |
|
|
| VS | EVA |
|
|
← Swipe →
Product Safety Information
1. Medical applications
Please do not use this product for permanent implantation in the human body or for medical applications involving a state of permanent contact with bodily fluids or human tissue (in this case, permanent means 30 days or more.)
Additionally, please consult with Dow-Mitsui Polychemicals in advance if you wish to use the product for other medical applications, such as for a medical device as defined in the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices.
2. Food packaging applications
Please contact Dow-Mitsui Polychemicals regarding the state of inclusion in (conformity to) domestic and overseas laws or regulations regarding food utensils, containers and packaging materials.
